We know there’s a growing shortage of and reduced access to physicians in the U.S. Just when we thought the pandemic was easing, new COVID-19 variants are emerging that will likely cause the physician access situation to worsen again. Fortunately, in an increasing number of states, pharmacists have been granted authority to prescribe some medications, as discussed in our recent blog on clinical protocol development and prescriptive authority.
As noted, pharmacists are highly accessible and are reported to be visited by patients 12 times more frequently than the patient’s primary care physician. Growing recognition that pharmacists can help fill unmet public health needs through their accessibility and medication knowledge has led to the adoption of statewide protocols for independent pharmacist prescriptive authority expanding the scope of pharmacy practice. Statewide protocols permit pharmacists with qualifying criteria to prescribe specific medications or medication categories.
So, we know the why and the who. There’s a shortage of physicians which reduces access to providers and medications. And, pharmacists, guided by scope-of-practice laws and standardized protocols established by state legislatures and regulated by appropriate boards, are in a unique position to help address that shortage.
Now, let’s look at the how, what and where.
1. How are statewide pharmacist prescribing protocols created?
Statewide prescribing protocols and standing orders are designed to increase access to healthcare and medications by utilizing pharmacists’ extensive medication knowledge, community accessibility, and the public’s trust of the profession. They address known public health problems that don’t require a diagnosis or for which a diagnosis requiring low-risk medications already exists. They are predictable and consistent, helping provide greater continuity of care and use of standardized practices. Statewide protocols don’t require collaborative practice agreements between a pharmacist and healthcare provider. Their authority is ratified by the state, and protocols are established by Boards of Pharmacy. Pharmacists may have to obtain additional licensure, complete specific continuing education, and/or notify primary care physicians following treatment. These statewide protocols help create continuity of care within a state. But, in an effort to create standardization between states, the National Alliance of State Pharmacy Association’s (NASPA) and the National Association of Boards of Pharmacy (NABP) have assembled the Statewide Protocol Workgroup. Their goal is to draft policy recommendations and develop templates for creating standardized statewide protocols for pharmacist prescriptive authority. Like protocols, statewide standing orders are another means of permitting pharmacists with autonomous prescribing abilities. Statewide standing orders detail various actions for pharmacists to follow when treating specific patient conditions. They’re signed by a state’s public health officer; however, unlike a protocol, they must be reimplemented and re-signed should a new public health officer assume the role in a given state.2. How do pharmacist prescribing regulations differ by state?
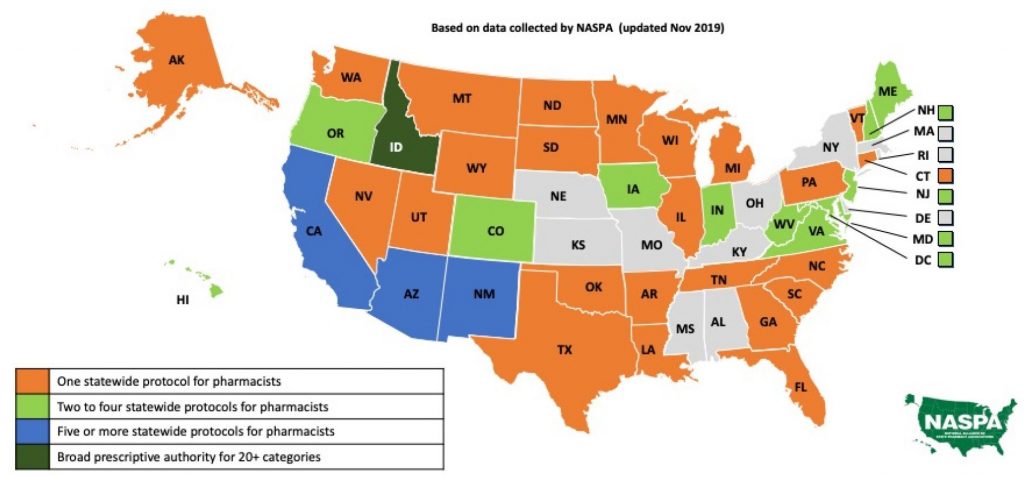
Statewide pharmacist prescriptive authority is continually growing as U.S. states introduce legislation and adopt protocols. Statewide protocols are the least restrictive, granting access to all qualified pharmacists within a state. In addition to statewide protocols, pharmacists have prescribing capabilities under collaborative practice agreements (CPA) or advanced certification as allowed by state laws. These further expand a pharmacist’s scope of practice; however, they are more restrictive and specific to only those pharmacists who seek the required CPAs or certifications. The most-common statewide protocols are for immunizations, naloxone, hormonal contraception, and smoking cessation. A few states have started to introduce additional pharmacist protocols for travel medications, fluoride, HIV pre- and post-exposure, and self-limiting medical conditions with low treatment risks.
3. Which states have statewide progorcols for pharmacists?
- Hormonal contraceptives — Pharmacists can prescribe hormonal contraceptives in Arizona, Arkansas, California, Colorado, District of Columbia, Hawaii, Idaho, Maryland, Minnesota, Nevada, New Hampshire, New Mexico, Oregon, Utah, Vermont, Virginia and West Virginia.
- Tobacco-cessation aids — Pharmacists can prescribe tobacco-cessation therapy in Arizona, Arkansas, California, Colorado, Indiana, Iowa, Oregon, Maine, Minnesota, Missouri, New Mexico, Vermont, West Virginia and Wyoming.
- Naloxone — Pharmacists have statewide prescriptive authority in 18 states, and 12 states have standing orders. All 50 states have some form of access to naloxone via pharmacist prescriptive authority or non-prescription availability.
- Immunizations — Pharmacists are permitted to administer immunizations in all 50 states under statewide protocol, standing orders, or pursuant to a prescription with some limitations to vaccine type or patient age.
- Other statewide protocol categories include:
- Colorado, Oregon — HIV Pre-Exposure Prophylaxis (PrEP) and Post-Exposure Prophylaxis (PEP). Other states have standing orders or laws allowing conditional pharmacist prescriptive authority for PrEP and PEP, such as California and New Jersey.
- California, New Mexico, Idaho — Travel medications.
- Virginia and Idaho — Minor, self-limiting medical conditions; epinephrine auto injectors; and fluoride supplements. Idaho allows pharmacists to follow current clinical guidelines for specified conditions in lieu of statewide protocol creation.
Contents of statewide protocols
The NASPA and NABP Statewide Protocol Workgroup recommend that statewide pharmacist protocols include:- The medications or categories of medications.
- Required pharmacist training or qualifications.
- Procedures regarding:
- Patient inclusion criteria.
- Documentation and record maintenance requirements.
- Communication requirements.